Clinical Data Coordinating Center
IMS provides coordinating center services for a variety of multi-center research efforts, including genomics projects, clinical trials, regional cancer surveillance data collection programs, and others.
Specific coordinating center tasks vary depending on the nature of the study. Components may include the collaborative development of protocols, procedures, and data collection tools to be used across participating centers, site training, assurance of regulatory and human subjects research compliance, development and application of quality assurance/quality control measures, and oversight of study conduct across participating centers. Additional responsibilities include the preparation of final merged data files across data sources, data file annotations, and analytic and reporting functions.
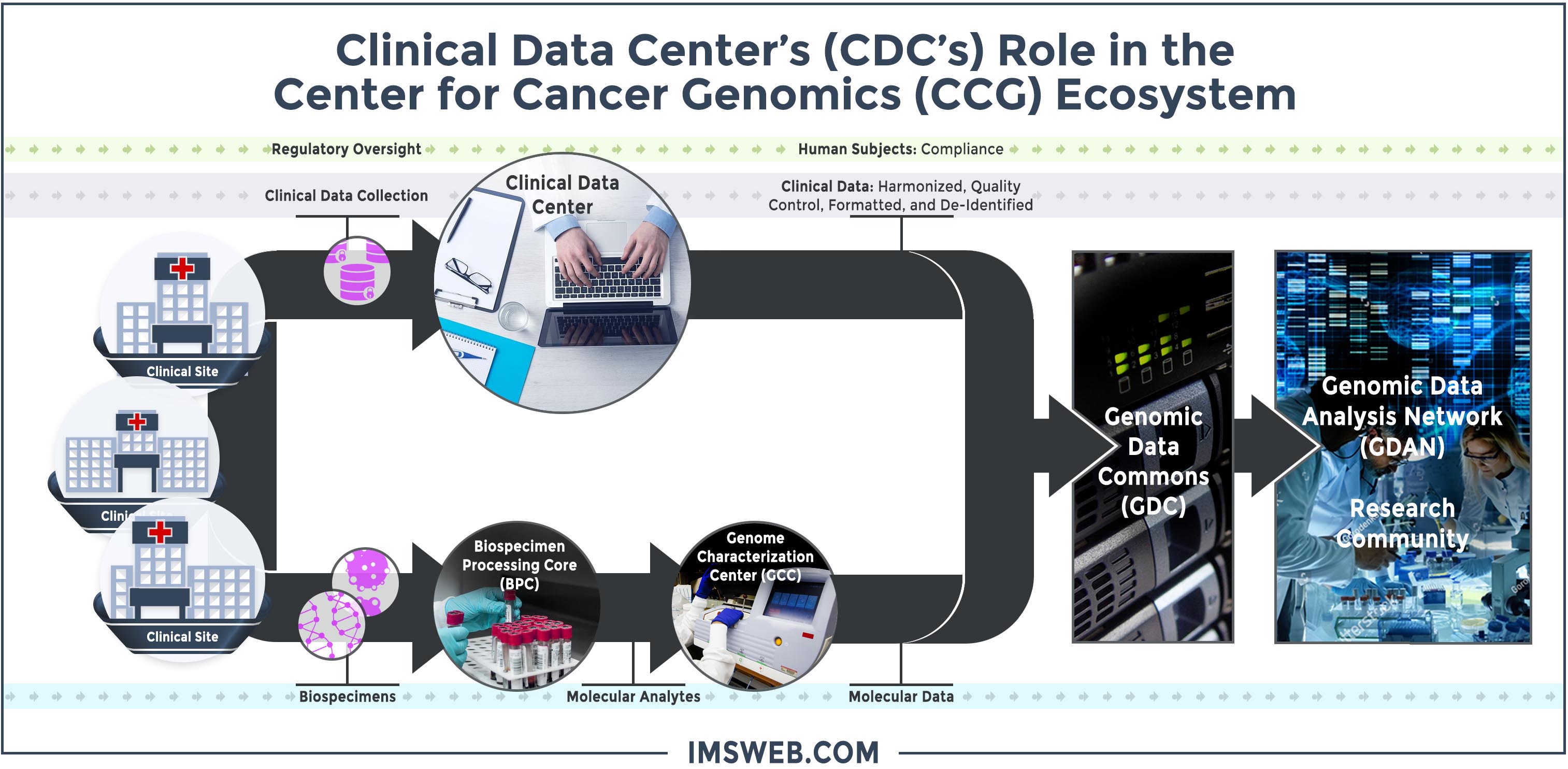
As an example, IMS serves as the Clinical Data Center (CDC) for the Center for Cancer Genomics (CCG). The CCG projects integrate molecular data with clinical data from patient donors and make data sets widely available for sharing with the research community. Participants in the CCG ecosystem are illustrated below. IMS facilitates communications between the diverse contributing entities to provide regulatory and human subjects oversight and data collection, quality control, harmonization, annotation, and de-identification services to create complex merged data files for distribution to the research community via the Genomic Data Commons (GDC).
Flowchart Description: Data from clinical sites is sent to the Clinical Data Center (CDC) which provides regulatory oversight and clinical data collection services. The CDC verifies human subjects compliance for the clinical data and also harmonizes, quality controls, formats, and de-identifies the data before sending it to the Genomic Data Commons (GDC). In a parallel process, biospecimens from the clinical data sites is sent to the Biospecimen Processing Core (BPC) which sends molecular analytes to the Genome Characterization Centers (GCC) for sequencing. The molecular data is also sent to the GDC. Clinical and molecular data at the GDC is made available to the Genome Data Analysis Network and the scientific community at large for analysis. View Larger Image
IMS Services
IMS provides a broad range of services for our clients, including but not limited to: Data Coordinating Centers; Clinical Trials Support; Data Hosting and Analysis; Web and Application Development and Hosting; Software As A Service (SaaS); and others.
Choose one of our services below to learn more about what we offer at IMS: